Fast Answers.
Meaningful Insights.
Pathlight, the ultra-sensitive MRD platform from SAGA, provides earlier detection to inform patient-provider decisions.
Tumor-informed and patient-specific
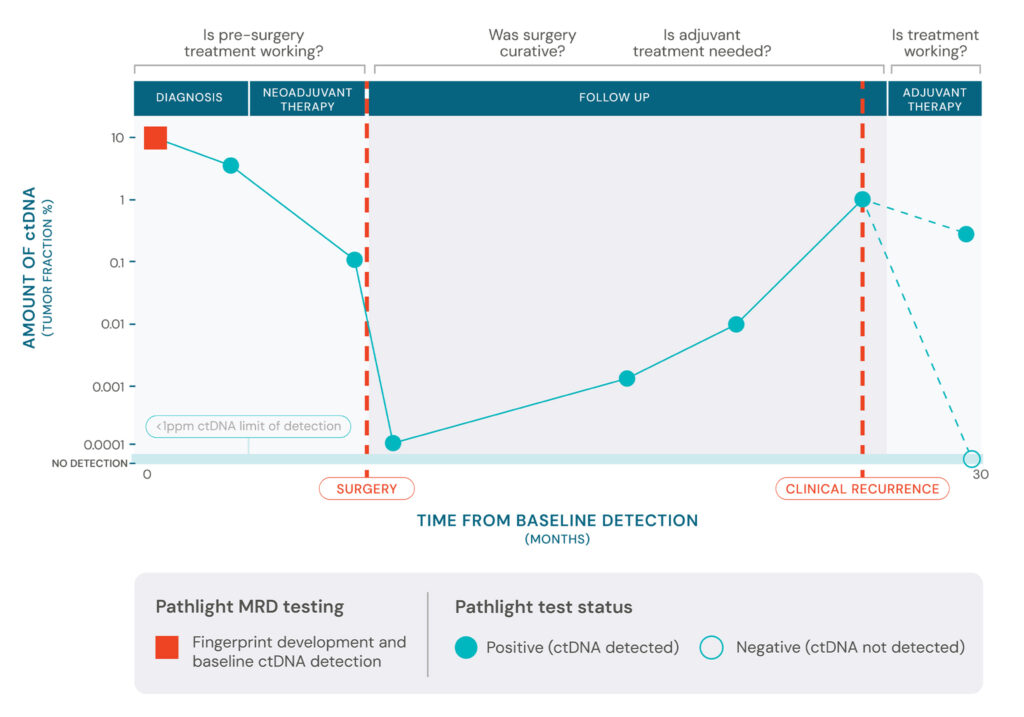
Clinicians need a reliable way to detect early molecular recurrence for monitoring neoadjuvant treatment; to perform risk stratification to guide decisions about adjuvant or extended endocrine therapy; and to assess evidence of residual disease post-surgery—providing patients with reassurance or guiding effective intervention.
Patients want peace of mind to know if pre-surgery treatment is working, whether surgery was curative or if additional treatment is needed.
First-generation MRD tests can fall short on reliably answering these questions because they rely on detecting single nucleotide variants (SNVs), which are susceptible to technical noise, leading to false positives that impact specificity and false negatives that decrease sensitivity, leading to shorter lead times across many tumor types.
Using ultra-sensitive MRD detection to inform recurrence monitoring and treatment response
Every cancer diagnosis comes with unknowns. Pathlight delivers trusted insights that enable better informed treatment decisions by physicians and patients.
Personalized tumor fingerprint development and initial blood test
Results will be available 3-4 weeks from receipt of both tissue and blood at the laboratory.
Subsequent blood tests
Results will be available 3- 5 days from receipt of blood at the laboratory.
Robust performance across multiple solid tumor types
Proven in clinical studies including top-tier pharma companies and leading US cancer centers

