Our Science
Addressing one of the most difficult challenges in cancer diagnostics.
Shining a light on a new era of cancer care
Pathlight, the company’s flagship blood-based, multi-cancer MRD platform, overcomes one of the toughest challenges in oncology – sensitive, specific, and earlier detection of molecular residual disease (MRD) and recurrence – increasing the chances for successful treatment.
Unparalleled specificity
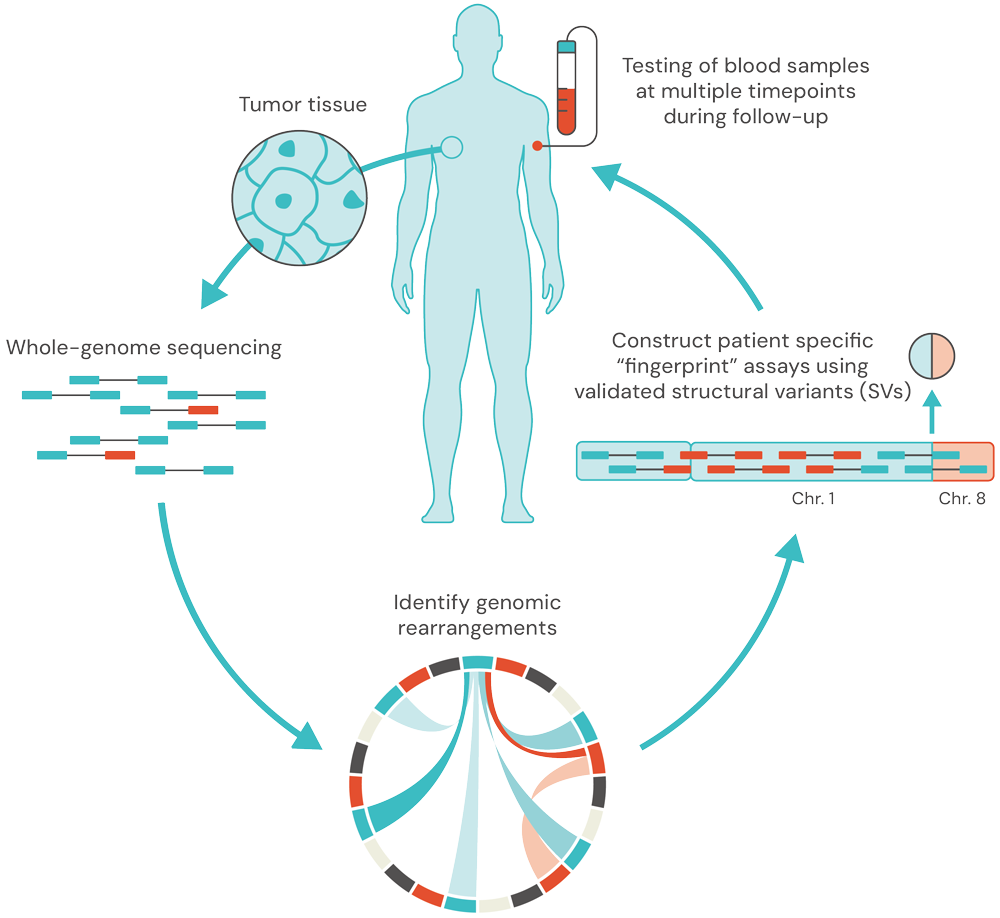
Structural Variants (SVs) are stable and unique to each patient and tumor, enhancing the specificity of MRD testing and reducing false positives.
Ultra-sensitive detection
SVs often occur early in tumorigenesis and are often amplified in the tumor, resulting in more ctDNA fragments, enabling sub 1ppm detection, 100% analytical specificity , and longer lead times.
No sensitivity cliff
NGS-based technologies have background error rates that determine what is called “positive.” Due to whole genome SV biology and SAGA’s proprietary technology platforms, Pathlight has no such threshold, meaning a positive result can be reported based on the detection of a single ctDNA molecule.
MRD testing for every stage
Whether for early-stage recurrence detection, late-stage metastatic monitoring, or tracking therapeutic response, Pathlight provides insights, empowering clinicians to make informed treatment decisions.
Visit the Pathlight Website
Posters and Publications
Our innovative research is regularly featured in leading journals and at major conferences. We are proud to share the progress of our work to address one of the most difficult challenges in cancer diagnostics: early, sensitive, and specific MRD testing.

References
1. Elliott MJ, Howarth K, Main S, et al. Ultrasensitive detection and monitoring of circulating tumor DNA using structural variants in early-stage breast cancer. Clin Cancer Res. Published online January 7, 2025. doi:10.1158/1078-0432.CCR-24-3472